Novel molecular tactics in the battle between killer lymphocytes and cancer
Our innate immune systems protect us against harmful and infectious pathogens. Pyroptosis is one form of cell death used to kill infected host cells. At the molecular level, pyroptosis is initiated by caspases in infected cells. Caspases activate gasdermin (GSDM) proteins which puncture the membrane of infected cells. Cytosolic contents are then released through these GSDM pores to activate additional immune cells, which prevent pathogens from further spreading throughout our bodies.
In new research, JCC Fellow Dr. Shiyu Xia and colleagues have shown that pyroptosis is also important in anti-tumor immunity. Killer lymphocytes deliver granzymes into cancer cells to activate GSDM proteins in cancer cells, causing pyroptosis. As pyroptosis boosts anti-tumor immunity, cancer cells have evolved strategies against pyroptosis. In new research published in Science Immunology, Dr. Xia discovered that one such strategy is alternative RNA splicing.

Dr. Xia studied GSDM proteins as a PhD student in Dr. Hao Wu’s lab. There are six GSDM proteins in humans, named GSDMA-F. Xia used a combination of biochemistry, structural biology, and cellular biology approaches to understand the molecular mechanisms of GSDMA, GSDMD, and GSDME. The newly published study is on the role of GSDMB in anti-tumor immunity. This study is a collaboration with Dr. Zhibin Zhang’s lab, and Dr. Qing Kong is a co-first author.
Gasdermin isoforms have opposing contributions to anti-tumor immunity
During Dr. Xia’s PhD studies, it was becoming increasingly clear that GSDM proteins may also influence cancer. However, there were contrasting reports as to whether GSDM proteins such as GSDMB suppress or activate tumor growth.
Dr. Xia predicted that part of this confusion may arise from collectively studying the functions of different GSDMB variants. RNA splicing generates at least 5 different GSDMB protein isoforms. Most GSDMB variants are co-expressed in cancer cells. By investigating the different variants, Xia discovered that only two variants (isoforms 3 and 4) are capable of pore formation in cellular membranes and crucial for triggering pyroptosis. Interestingly, the isoforms that do not form pores on their own lower the pore-forming activity of isoforms 3 and 4. Thus, cancer cells likely prevent pyroptosis by expressing alternatively spliced GSDMB variants.

GSDMB isoforms and their distinct toxicities (adapted from Fig. 1)
Gasdermin isoform expression in bladder and cervical tumors correlates with clinical outcomes
Next, Dr. Xia examined the expression of GASDB isoforms in bladder and cervical tumors. He found that the expression of the pore-forming isoforms 3 and 4 was associated with better patient outcomes. In contrast, expression of isoforms 1 and 2, which do not form pores, are not associated with better patient outcomes. Thus, Xia and his colleagues think that overexpression of the non-pore forming isoforms may help cancer cells to evade pyroptosis. Furthermore, Xia predicts that the selective modulation of gasdermin isoform expression (upregulating pore-forming isoforms and downregulating pore-inhibiting isoforms) may be one way to treat cancer in the future.
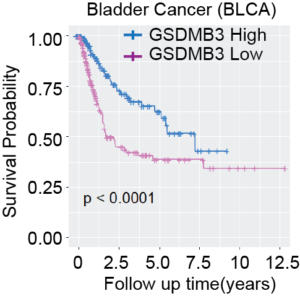
High expression of GSDMB3 correlates with better survival of bladder cancer patients (adapted from Fig. 3)
Future steps: a synthetic approach to engineer anti-tumor immunity
Now that Dr. Xia has a better understanding of natural pathways that modulate anti-tumor immunity, he is working towards engineering synthetic pathways – “circuits” – as smart programmable immunotherapies. This is a reason why he joined Dr. Michael Elowitz’s lab, who is an expert in synthetic biology. These synthetic circuits operate orthogonally to, as well as interface with naturally occurring pathways. Therefore, the use of such circuits will greatly expand biological interventions beyond what currently exists within natural systems.