Molecular changes in aging skin influence speed of wound healing
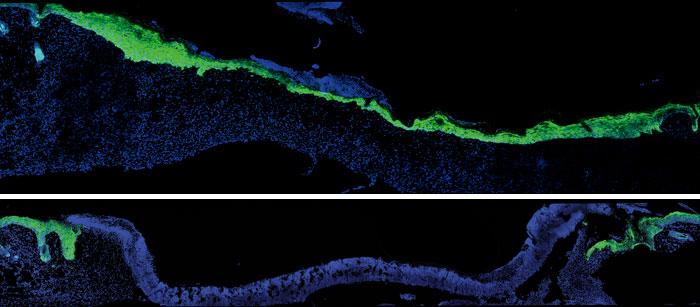
Caption: Five days after an injury to young mouse skin (blue), new skin cells (green) fill the wound (top). When researchers turned down expression of a protein skin cells use to talk to nearby immune cells, new skin took much longer to arrive (bottom). Image credit: Laboratory of Mammalian Cell Biology at The Rockefeller University/Cell
Age-related slowdown in wound healing is due to a loss of communication between epithelial and immune cells in the skin, according to recent research co-led by JCC Fellow Siqi Liu (2016-2018).
Wound healing requires that skin cells move in, coordinate with immune cells, and close the injured skin. Liu and her coauthors compared wound healing in two-month-old versus 24-month-old mice — roughly equivalent to 20- and 70-year-old humans. After a scab formed, new skin cells called keratinocytes formed a sheet to fill in the wound under the scab. But among older mice, keratinocytes migrated under the scab more slowly, did not proliferate very well, and could not activate immune cells (DETCs), leading to delayed wound closure.
Investigating the mechanism behind these changes, the researchers found that aged keratinocytes at the wound edge did not upregulate or activate specific proteins known as skints and STAT3. Next, in separate experiments on young mouse skin, they interfered with immune cells, skints, and STAT3 and found that wound healing was disrupted.
“Together with another postdoctoral fellow Brice Keyes, I provided genetic evidence showing that a family of genes named Skint proteins are important for skin wound healing,” explains Liu, who studies in the laboratory of HHMI Investigator Elaine Fuchs at The Rockefeller University. “They regulate the function of skin resident T cells (DETCs) during wound healing and their expressions are controlled by a upstream transcription factor Stat3. When we genetically ablated Stat3, Skint or DETCs specifically in young mouse skin, cutaneous wound healing was delayed.”
Could researchers reverse this deterioration in signaling to immune cells? Knowing that immune cells at the wound site normally release a different protein to aid healing, researchers applied this protein to mouse skin in petri dishes. Keratinocyte migration increased, in effect making the skin behave more youthfully. The results were published in Cell.
As a biochemistry graduate student, Liu studied innate immune signaling pathways in vitro. “One of my biggest research challenges as a postdoc has been the transition from focusing on specific signaling pathways in vitro to studying complex biologic processes in vivo using mouse models,” says Liu. “The signaling pathways that coordinate tissue repair in animals are far more complex than those isolated in a test tube or cultured cells. There is much more cross-talk and plasticity between different signaling components in vivo. For example, in our paper, we found multiple intrinsic and extrinsic factors contributing to the delayed wound healing in aged mice, rather than a single cause. My training and background have encouraged me to think like a biochemist when studying complex biological processes and to focus on a specific aspect of skin wound repair.”
Liu, who intends to seek an academic position following her postdoc, adds, “Aging skin provided a robust animal model to study wound repair because aged skin has compromised healing abilities compared to that of its younger counterpart. Our findings could provide insights into the development of therapeutics to accelerate age-related decline in wound healing.”